Medical
-
Raising the level of quality and efficiency of medical instrument traceability and maintenance to ensure the safety of patients.
Outline of Medical Business
Roland DG has concentrated its efforts in the medical field in providing solutions that meet Unique Device Identification (UDI) regulations requiring permanent identification information on medical devices and instruments. In recent years, efforts to review medical instrument safety and management procedures have been increasing in order to reduce the risk of infection inside hospitals and ensure the safety of patients. Regulatory bodies worldwide are seeking a comprehensive management system for instruments and devices in use at medical facilities.
Roland DG recognized an opportunity to apply its dot impact printing technology, which had been cultivated since the early 2000s, to produce marking identifiers for medical devices and instruments. In anticipation of this requirement, the company released the MPX-90M medical instrument marking device in 2012 followed by the MPX-95 with Direct Part Marking (DPM) kit in 2016. The devices enable medical facilities as well as medical instrument manufacturers and suppliers worldwide to utilize a 2D symbol imprinted on each instrument as a permanent solution for tracking available stock, location, and usage history.
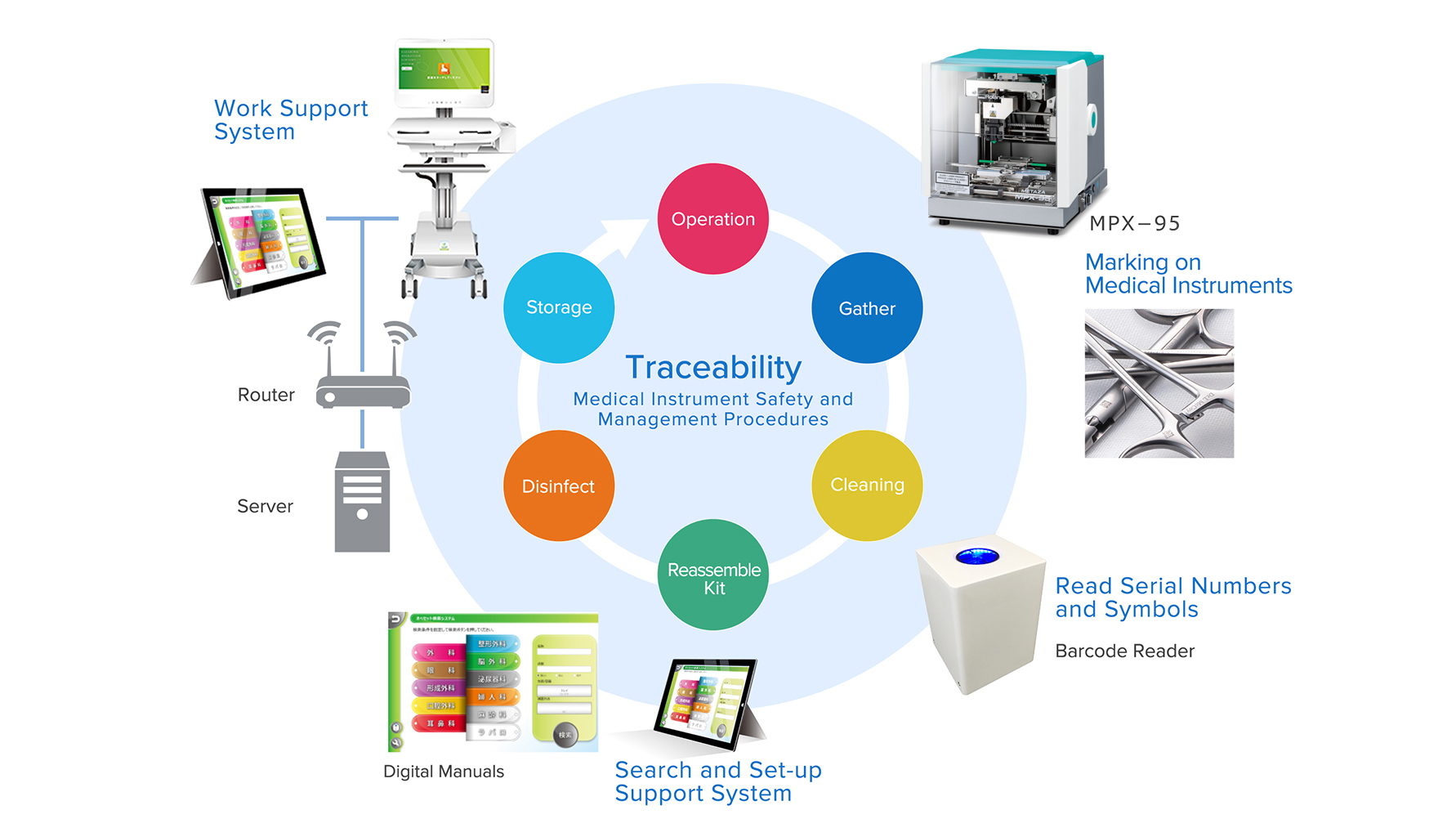
Roland DG also envisioned that medical facilities could incorporate proven digital support systems currently in place at Roland DG factories to raise the level of quality and efficiency involving pre- and post-operation maintenance tasks. The cleaning, disinfection and reassembly of medical instrument kits include complicated procedures across a wide variety of surgical tools that are highly dependent on employee experience. Today, DGSHAPE is working with Hamamatsu University School of Medicine to develop and provide a proof of concept for a computer-driven medical instrument traceability and maintenance support system. DGSHAPE intends to begin offering a comprehensive solution to medium to large-scale clinics and hospitals in Japan and overseas within 2017.
-
Medical Instrument Marking Devices
The medical instrument marking device is a desktop-size dot impact printer designed for the direct marking of medical instruments such as scalpels and forceps with UDI (Unique Device Identification) barcodes for tracking and traceability. It quickly creates 2D DataMatrix barcodes to GS1* standards and imprints them with great precision on the surface of medical instruments in areas as small as one square millimeter. The machine uses dot pin marking to permanently mark surfaces and prevent erasure and corrosion. A vise secures the instrument while a laser pointer indicates the area to be marked so that no special training is required.
* The GS1 DataMatrix is a standard for 2D symbols determined by the GS1 international body for barcodes, symbols and electronic data transfer, and it provides worldwide electronics manufacturers and medical facilities a way to display symbols on instruments. Information on item code, usage limits, lot number, serial number and more can be stored within 26 bytes and read using a special scanner.
Applications
-
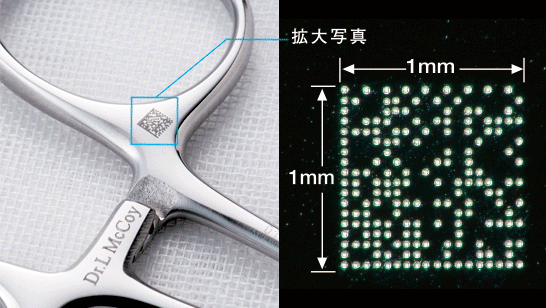
2D Symbol Imprinted on Medical Instruments -
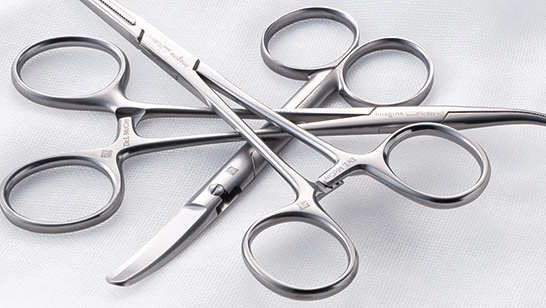
Imprinted Forceps -

Imprinted Tweezers
Medical Instrument Safety and Management Procedures